Alzheimer’s disease research has taken a groundbreaking turn with the pioneering work of neuroscientist Beth Stevens. Her innovative approach focuses on understanding the role of microglial cells, integral components of the brain’s immune system, in the development of neurodegenerative diseases such as Alzheimer’s. By analyzing how these cells interact with synapses, Stevens has uncovered the intricate balance between neural connections and immune responses, which can lead to critical insights into Alzheimer’s treatments. As the population ages, the prevalence of Alzheimer’s is projected to rise drastically, highlighting the urgency for effective interventions. Stevens’ findings not only pave the way for novel therapeutic strategies but also set the stage for earlier biomarkers that could revolutionize diagnosis and treatment for millions.
The exploration of Alzheimer’s disease, a devastating form of dementia, is increasingly becoming a focal point in modern medical research. Delving into the complexities of neurodegeneration, scientists like Beth Stevens are unearthing the vital roles that brain immune cells, or microglia, play in maintaining neural health. These guardians of the brain actively manage synaptic connections and respond to injury, but their dysfunction is linked to neurodegenerative conditions. The promising discoveries surrounding these cellular interactions provide hope for developing better treatments and improving outcomes for those affected. As the quest to understand and combat Alzheimer’s evolves, advances in this area could significantly enhance our knowledge of the brain and its susceptibility to disease.
Understanding Microglial Cells in Alzheimer’s Disease
Microglial cells play a crucial role in maintaining brain health, acting as the main immune defense in the central nervous system. These cells are constantly patrolling for signs of injury or disease, ensuring that the neural environment remains stable. Their ability to prune synapses—an essential function for learning and memory—has been central to recent Alzheimer’s disease research. However, when this process goes awry, it may lead to neurodegenerative conditions such as Alzheimer’s, where excessive pruning can diminish synaptic connections critical for cognitive function.
Recent studies led by Beth Stevens and her team have illuminated the dual nature of microglial cells. While they protect the brain from pathogens and clear out cellular debris, dysfunction in these cells can exacerbate the symptoms of Alzheimer’s. Accordingly, understanding the underlying mechanisms of microglial behavior has opened new avenues for developing potential Alzheimer’s treatments. Identifying biomarkers that signal microglial dysfunction may allow for earlier detection and intervention, significantly improving outcomes for patients.
The Transformative Impact of Beth Stevens’ Research
Beth Stevens’ groundbreaking research has shifted the paradigm on how we perceive the role of microglial cells in neurodegenerative diseases. As an associate professor at Harvard Medical School, her work has revealed that these brain immune cells are not merely passive defenders; they actively shape the brain’s neural networks during both normal development and disease states. By establishing a connection between improper synaptic pruning and conditions like Alzheimer’s, Stevens has paved the way for developing innovative therapies aimed at correcting these harmful processes.
Moreover, Stevens emphasizes the importance of basic scientific research in uncovering new pathways for treatment. Her work highlights that foundational studies, often seen as distant from clinical applications, are critical for later therapeutic advances. By funding and fostering curiosity-driven science, Stevens and her lab have made strides that could lead to transformative Alzheimer’s treatments, potentially altering the trajectory of this devastating disease.
Funding and Support for Alzheimer’s Research
The journey of Alzheimer’s disease research, particularly in the domain of microglial function, is significantly supported by federal funding, as mentioned by Beth Stevens. The National Institutes of Health (NIH) has been instrumental in providing the necessary resources to study the complexities of the brain’s immune system. This support allows for the exploration of neurodegenerative diseases at a fundamental level, which is essential for developing innovative treatments that translate into real-world benefits for patients.
Without such financial backing, many of the breakthroughs in understanding microglial roles and their impact on Alzheimer’s could remain undiscovered. Stevens’ experience underscores the vital connection between basic research and potential clinical applications, advocating for continued investment in science that might initially seem disconnected from immediate health outcomes. The advancements in understanding the brain’s immune system could lead to breakthroughs that reshape the landscape of Alzheimer’s care.
Neurodegenerative Diseases: The Broader Perspective
While Alzheimer’s disease remains a primary focus, research on microglial cells contributes to a broader understanding of various neurodegenerative diseases. Conditions such as Huntington’s disease and multiple sclerosis exhibit similar immune response anomalies that can be traced to microglial functionality. By investigating how these cells operate in different contexts, researchers can unearth universal mechanisms that might be targeted therapeutically, providing insights applicable to multiple diseases beyond just Alzheimer’s.
The exploration of microglial cells within different neurodegenerative frameworks widens the scope of treatment options. By identifying common pathways in disease manifestation, scientists could potentially develop multi-target therapies. This integrative approach not only enhances the understanding of Alzheimer’s and its coexistence with other disorders but also strengthens the scientific foundation for innovative Alzheimer’s treatments, which can ultimately benefit a larger patient population.
The Importance of Basic Science in Disease Research
Beth Stevens’ statement regarding the significance of basic science highlights a crucial aspect of medical research—the need for foundational discoveries that may not provide immediate clinical benefits. Basic science forms the bedrock of our understanding of complex systems, such as the brain’s immune responses. This knowledge is vital for diagnosing and treating diseases like Alzheimer’s, where understanding the microglial cells’ activities can lead to breakthroughs that directly impact patient care.
The journey from basic scientific inquiry to clinical application often takes many twists and turns, but it is these explorations that yield the most transformative insights. Researchers like Stevens demonstrate how curiosity-driven investigation can lead to unexpected yet groundbreaking advancements in treating neurodegenerative diseases, reinforcing the value of investing in basic research to fight against Alzheimer’s and similar disorders.
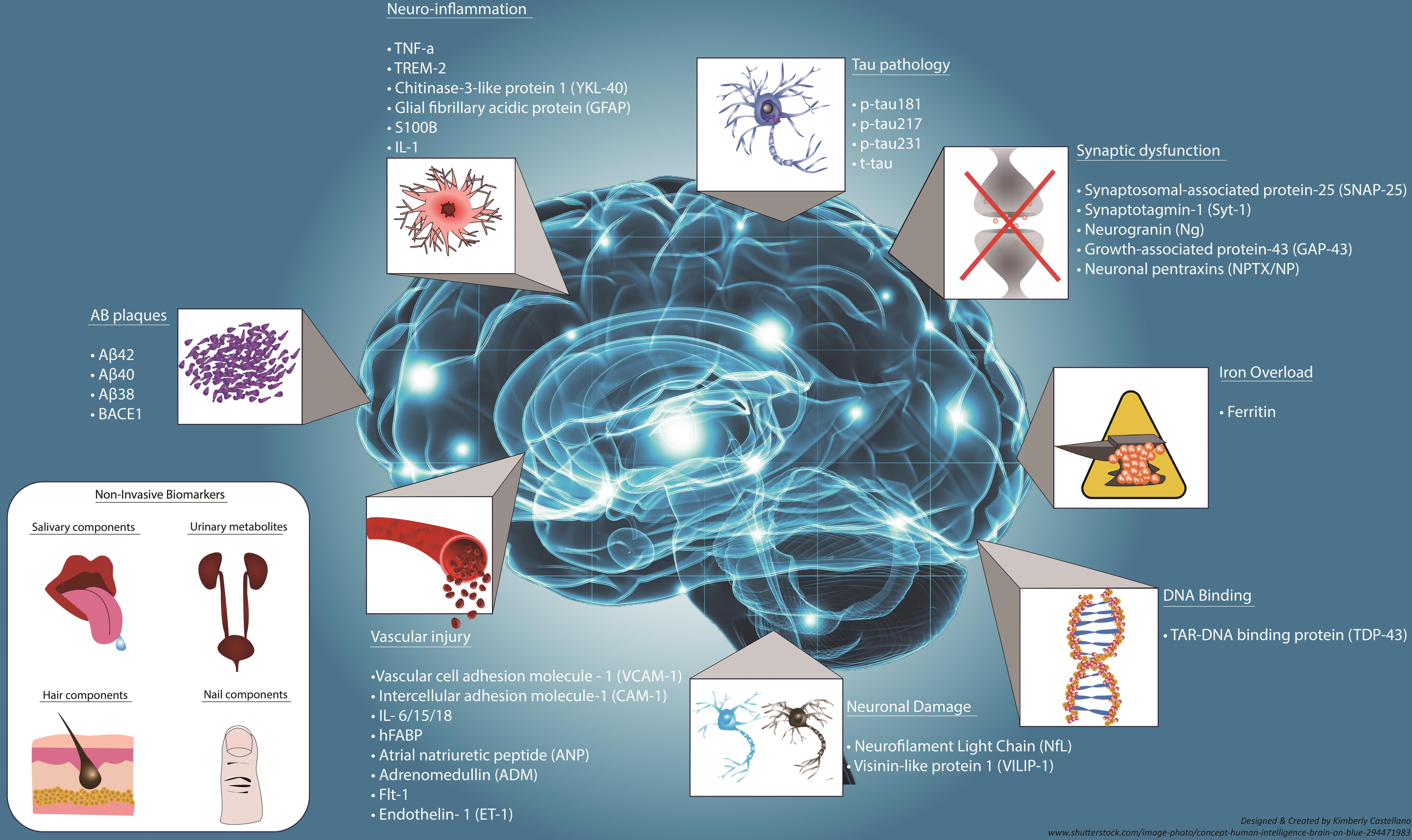
Early Detection and Biomarkers in Alzheimer’s Research
The quest for early detection of Alzheimer’s disease is accelerated by research into biomarkers associated with microglial function. Beth Stevens’ work illustrates how abnormalities in microglial activity can serve as indicators for the onset of Alzheimer’s, paving the way for earlier diagnosis. Identifying these biomarkers is crucial, as early intervention may significantly alter the course of the disease, allowing for treatments that help maintain cognitive function longer.
Biomarkers related to microglial health could lead to non-invasive diagnostic techniques, which would be a significant milestone in Alzheimer’s research. This approach not only enhances the potential for timely therapeutic intervention but also encourages a proactive stance in monitoring brain health, fostering a new era of personalized medicine for neurodegenerative diseases.
Innovations in Alzheimer’s Treatments: A Future Perspective
The innovative research spearheaded by Beth Stevens and her lab gives hope for future Alzheimer’s treatments that could fundamentally alter the progression of the disease. By understanding how microglial cells contribute to synaptic pruning and neuronal health, scientists are moving towards therapies that enhance the brain’s immune responses. These treatments could potentially mitigate the damaging effects of improper pruning and improve cognitive functions in patients.
As the demand for effective Alzheimer’s treatments increases due to an aging population, the insights gained from microglial research may lead to the development of targeted therapies that work at the cellular level. By continuing to explore the brain’s immune systems and their role in neurodegeneration, researchers are establishing a robust framework for creating effective interventions that can improve the lives of millions suffering from Alzheimer’s and similar diseases.
Community Impact and Alzheimer’s Awareness
As researchers like Beth Stevens advance our understanding of Alzheimer’s disease and its mechanisms, raising community awareness becomes essential. The more informed the public is about the disease, the greater the support for funding research initiatives and programs aimed at combating neurodegenerative diseases. Engaging with communities through educational outreach can demystify the complexities of Alzheimer’s, encouraging early detection practices and fostering a supportive environment for affected families.
Increased awareness also plays a vital role in advocacy for policy changes regarding Alzheimer’s funding and resources. By sharing findings from research on microglial cells and their role in diseases like Alzheimer’s, advocates can rally support for legislative changes that prioritize funding for this crucial area of study. This collaborative effort can drive significant advancements in the research landscape, ultimately leading to better outcomes for patients and families coping with the challenges of Alzheimer’s.
The Future of Alzheimer’s Research: Collaboration and Innovation
The future of Alzheimer’s disease research hinges on collaboration and interdisciplinary approaches, bringing together scientists from various fields to tackle this complex neurodegenerative disorder. Efforts like those seen in Stevens’ lab highlight the importance of merging insights from immunology, neurology, and clinical sciences to enrich our understanding of microglial cells and their implications in Alzheimer’s. Such collaboration can unlock novel pathways for research and development of Alzheimer’s treatments.
As we move forward, embracing innovation and novel research techniques will be paramount in addressing the challenges posed by Alzheimer’s disease. The integration of cutting-edge technologies, such as advanced imaging and genetic analyses, will enhance our ability to observe microglial function in real-time, unveiling critical information necessary for identifying effective interventions. Through collective efforts, the ultimate goal remains clear: to mitigate the devastating impact of Alzheimer’s and improve the lives of those affected by this condition.
Frequently Asked Questions
What is the role of microglial cells in Alzheimer’s disease research?
Microglial cells are essential components of the brain’s immune system, playing a crucial role in Alzheimer’s disease research. They help to clear damaged cells and prune synapses within the brain. Recent studies have shown that abnormal microglial activity can contribute to neurodegenerative diseases like Alzheimer’s by improperly pruning synaptic connections, which can lead to cognitive decline.
How does Beth Stevens contribute to Alzheimer’s disease research?
Beth Stevens is a prominent neuroscientist leading groundbreaking research on microglial cells, highlighting their role in the brain’s immune response related to Alzheimer’s disease. Her work investigates how these cells contribute to synaptic pruning and how disruptions in this process can lead to neurodegenerative diseases, paving the way for potential Alzheimer’s treatments and early detection biomarkers.
What are the implications of aberrant microglial pruning in neurodegenerative diseases?
Aberrant microglial pruning is implicated in various neurodegenerative diseases, including Alzheimer’s. It can lead to the loss of critical synaptic connections, affecting communication between neurons and resulting in cognitive deficits. Understanding this process is vital for developing effective treatments and interventions aimed at combating Alzheimer’s and similar conditions.
Why is funding from the National Institutes of Health important for Alzheimer’s disease research?
Funding from the National Institutes of Health (NIH) is crucial for advancing Alzheimer’s disease research. It supports foundational studies that investigate the roles of microglial cells and other aspects of neurobiology. This funding enables researchers like Beth Stevens to explore complex scientific questions that ultimately lead to discoveries that can transform our understanding of Alzheimer’s treatments and therapies.
What is the expected future impact of Alzheimer’s disease research on treatment options?
Research into Alzheimer’s disease, particularly studies focusing on microglial cells and neuroimmune interactions, is expected to significantly impact treatment options in the future. As scientists uncover more about the cellular mechanisms involved, new therapeutic strategies may emerge that could alter the course of the disease and improve the quality of life for millions affected by Alzheimer’s.
How can research on microglial cells improve early detection of Alzheimer’s disease?
Research on microglial cells offers insights into the immune processes within the brain that may be altered in Alzheimer’s disease. By identifying specific biomarkers linked to abnormal microglial activity, scientists hope to develop early detection methods. This could allow for interventions before significant cognitive decline occurs, drastically changing the prognosis for those at risk.
| Key Point | Details |
|---|---|
| Researcher | Beth Stevens, neuroscientist at Harvard Medical School. |
| Focus of Research | Transforming understanding of microglial cells’ role in the brain’s immune system. |
| Significance of Microglia | They clear out damaged cells and prune synapses, essential for neuron communication. |
| Impact on Diseases | Aberrant pruning linked to Alzheimer’s, Huntington’s, and other disorders. |
| Research Funding | Primarily supported by NIH and federal grants, essential for foundational studies. |
| Future Implications | Research may lead to new medicines and early biomarkers for Alzheimer’s disease. |
| Projected Trends | Estimated doubling of cases in the U.S. by 2050, increasing care costs significantly. |
| Personal Insight | Stevens emphasizes the importance of basic science for potential future applications. |
Summary
Alzheimer’s disease research is crucial as it explores innovative ways to combat the devastating effects of this neurodegenerative condition. By advancing our understanding of microglial cells, researchers like Beth Stevens are paving the way for new therapeutic strategies and early detection methods. With the potential to transform lives, ongoing studies promise to address the growing concern of Alzheimer’s, especially as the aging population increases the likelihood of diagnosis. Investing in such research is imperative for improving treatment outcomes and alleviating future healthcare burdens.